CMS Releases Proposed Rule for Long-Term Care Facilities
On July 16, 2019, the Centers for Medicare and Medicaid Services (CMS) released Requirements for Long-Term Care Facilities: Regulatory Provisions to Promote Efficiency and Transparency in a proposed rule to reform the Phase 3, Requirements of Participation (RoPs).
CMS has stated that through identification, examination, and industry and stakeholder input they identified:
Obsolete and burdensome regulations that could be eliminated and reformed to improve effectiveness or reduce unnecessary reporting requirements and other costs, with a particular focus on freeing up resources that health care providers, health plans and states could use to improve and enhance resident health and safety.
LeadingAge has done an initial review of the 110-page rule and outlined below a summary of the major proposed changes, edits, and revisions.
§483.10 Resident rights
Currently, nursing homes are expected to ensure that residents “remain informed” of the name, specialty, and way of contacting physicians and medical professionals involved in their care. Under the proposed rule, nursing homes will be relieved of the burden of ensuring that residents “remain informed” and will instead be required to inform residents on admission, upon any changes, and upon request of such information of the primary care physician only.
Several changes are proposed pertaining to grievances in this section. The proposed rule would add language to distinguish a grievance from “general feedback provided by the resident or their representative” and would remove detailed requirements for the responsibilities of the grievance official. The proposed rule would remove language pertaining to the required elements of a written grievance decision, though the expectation remains that much of this information would already be included “as a standard practice to ensure that the written decision is complete and informative.” The proposed rule would also change the retention period of evidence related to grievances from 3 years to 18 months.
§483.15 Admission, transfer, and discharge rights
Under the current regulatory requirement, nursing homes must send copies of all transfer and discharge notices to the state long-term care ombudsman. The proposed rule would require that copies of these notices are sent to the ombudsman only in cases of “facility-initiated involuntary transfers and discharges.” The proposed rule further notes that “this would not include residents who request the transfer or who are transferred on an emergency basis to an acute care facility when return is expected.”
§483.25 Quality of care
The language in current requirements refers to the “installation” of bed rails. Due to the inevitability of many beds being purchased and arriving in nursing homes with bed rails already in place, the proposed rule would remove all references to “installation” of bed rails and replace with “use” of bed rails. The expectations around appropriate use of bed rails would not change.
§483.35 Nursing services
Nurse staffing data is required to be posted daily in nursing homes to provide real-time information to residents and families about who is working and the amount of staff working in a nursing home during a given shift. Currently required to maintain this data for 18 months, the proposed rule would change this requirement to a retention period of 15 months.
§483.40 Behavioral health services
The requirements under this section remain largely unchanged and the changes introduced in the proposed rule relate to duplication of information. The rule proposes to remove the language stating that the facility must have sufficient staff who provide direct services to residents with the appropriate competencies and skills sets to provide nursing and related services to assure resident safety and attain or maintain the highest practicable physical, mental and psychosocial well-being of each resident, as well as removing language that any required rehabilitative services specified in the comprehensive care plan must be provided or obtained from an outside source, as both requirements are stated in other sections of the regulation (§483.35 Nursing services and §483.65 Specialized rehabilitative services, respectively).
§483.45 Pharmacy services
The proposed rule would make changes to this section in 2 key ways. First, the proposed rule would require that facilities’ policies, standards, and procedures pertaining to the use of psychotropic medications use recognized standards of practice, including circumstances under which PRN orders for psychotropic medications could be extended beyond the current 14-day limitation, and that the facility take into consideration individualized residents’ needs for psychotropic drugs.
The second proposal to this section relates to extending PRN medications past the 14-day limit. Currently, in order to extend a PRN psychotropic that is not an anti-psychotic past the 14-day limit, a physician must document in the medical record the rationale for the extension and indicate the duration for the PRN order. A PRN antipsychotic medication cannot be extended past the 14-day limit unless the attending or prescribing physician evaluates the resident for the appropriateness of the medication. The rule proposes that any extension of PRN psychotropic medications, including PRN antipsychotics, must, in addition to existing requirements, be in accordance with facility policy.
§483.60 Food and nutrition services
Current regulation sets forth extensive requirements for individuals serving as director of food and nutrition services if no qualified dietitian or other clinically qualified nutrition professional is employed full-time. These requirements include other certifications such as certified dietary manager, certified food service manager, certifications in food service management and safety, or an associates’ degree or higher in food service management or hospitality. The proposed rule would eliminate these requirements and instead allow nursing homes to designate as director of food and nutrition services an individual who has 2 or more years’ experience in this position or who has completed a course of study in food safety that includes topics such as foodborne illness, sanitation procedures, and food purchasing/receiving.
§483.70 Administration
In reviewing existing long-term care requirements, CMS has determined that while a facility assessment must be completed, certain requirements of the facility assessment are duplicated in other regulation. The facility-wide assessment for day-to-day operations and emergencies required under this section duplicates requirements under emergency preparedness to document facility-based and community-based risk assessments utilizing an all-hazards approach. As a result, the proposed rule would eliminate the requirement for a facility-wide assessment for day-to-day operations and emergencies with the understanding that a nursing home would still complete the assessments required under emergency preparedness.
Additional changes to this section include requiring the facility assessment to be completed at least every 2 years, as opposed to annually. CMS notes in the proposed rule that facilities may need to conduct a facility assessment more frequently and “in facilities with a high staff turnover, assessments should take place as frequently as necessary and the issue should be addressed in the QAPI plan.”
§483.75 Quality assurance and performance improvement
Many of the prescriptive requirements for QAPI program design and scope would be removed with the proposed rule, giving nursing homes the flexibility to determine how best to design the QAPI program to fit their individual needs and promote quality care. The proposed rule would also maintain requirements that facilities develop policies and procedures related to program feedback, data systems, and monitoring, but would eliminate the specific details of what these policies and procedures must include. The proposed rule would effect the same changes on program systematic analysis and systemic action, maintaining the requirement that facilities take action toward quality improvement, measure success, and track performance, but eliminating the overly prescriptive details of requirements for related policies and procedures.
§483.80 Infection control
As part of the Infection Prevention and Control Program (IPCP), a facility must designate one or more individuals to serve as infection preventionists who are responsible for the facility’s IPCP. Current requirements state that this individual must work at the facility at least part-time. The proposed rule would remove this requirement, requiring instead that the individual(s) designated as the infection preventionist(s) “must have sufficient time at the facility to meet the objectives set forth in the facility’s IPCP.”
§483.85 Compliance and ethics program
Though this section of the regulation has not yet been implemented, CMS has proposed to eliminate many of the requirements not currently in statute for a compliance and ethics program. The proposed rule would eliminate the requirement for annual review of the program and instead require the program to be reviewed “periodically.” While the requirement would be maintained that “high-level personnel” would be assigned with the overall responsibility to oversee compliance, the language suggesting that these individuals might include the chief executive officer, members of the board of directors, or directors of major divisions in the operating organization would be removed. For operating organizations with five or more facilities, the requirement for a compliance officer and a designated compliance liaison would be eliminated.
§483.90 Physical environment
With respect to life safety compliance, the rule proposes three significant changes to this section:
- CMS proposes to allow existing LTC facilities (certified before July 5, 2016) that had used the Fire Safety Evaluation System (FSES) for fire/life safety equivalency to continue to use the mandatory values specified in the 2001 edition of NFPA 101A.
- CMS proposes to revise §483.90(d) to require regular inspection of all bed frames, mattresses, and bed rails to identify possible entrapment hazards. When bed rails and mattresses are used and purchased separately from the bed frame, the facility must ensure that these components are compatible.
- CMS proposes to revise §483.90(e)(1)(i) regarding the number of residents per room and §483.90(f) for bathroom facilities to apply only to newly constructed facilities and newly certified facilities that have never been a long-term facility.
The proposed rule states that CMS recognized that the adoption of the 2012 edition of NFPA 101, Life Safety Code (LSC) and the referenced adoption of the 2013 edition of NFPA 101A and its mandatory values has resulted in some LTC facilities not being able to achieve passing scores in the FSES. Most notably the FSES was applied to LTC facilities that were built to construction types that do not comply with the construction type requirements for existing buildings in the LSC. These facilities had passing scores in the 2001 FSES based on protection throughout by automatic sprinklers.
The proposed NFPA 101A mandatory values for Extinguishment and People Movement in Table 1 of the draft rule appears to replace Worksheet 4.7.8B in the 2013 edition NFPA 101A. The proposed values match those in Worksheet 4.7.8 of the 2001 edition.
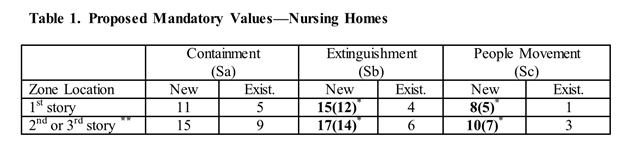

This proposed change, if enacted, would obviate the need for extensions of Time Limited Waivers (TLWs) obtained for construction type deficiencies, thereby providing much-needed relief to LTC facilities.
The proposed rule is now in the comment period until September 16, 2019. LeadingAge will continue to analyze and inform members of the intricacies of the proposal in addition to our draft comments prior to September. We encourage all members to submit comments to CMS or work with our Policy team to incorporate language and framework to support and/or improve comments.

Most Recommended
October 15, 2025
 Shutdown Week Three: Impact of Ongoing Closure on Affordable Housing
Shutdown Week Three: Impact of Ongoing Closure on Affordable Housing
February 24, 2026
Fiscal Year (FY) Funding 2026
October 07, 2025
Immigrant Workforce Matching Program Brings Workforce Relief
Recently Added
February 26, 2026
 Vance, Oz Announce Medicaid Funding Withheld From Minnesota
Vance, Oz Announce Medicaid Funding Withheld From Minnesota
February 25, 2026
CBO: HR 1 Speeds Medicare Part A Insolvency by 12 Years
February 24, 2026
DHS Proposes Asylum-Related Work Authorization Change
February 20, 2026