The provisions in the CAA 2021 direct the Secretary of Health and Human Services to create an SFP for poor-performing hospice programs, give authority for imposing enforcement remedies for noncompliant hospice programs, and require the development and implementation of a range of remedies—as well as procedures for appealing determinations regarding these remedies. CMS finalized all the CAA 2021 provisions related to hospice survey and enforcement in CY 2022 rulemaking except for the SFP.
LeadingAge advocated for the SFP to have clear entry and exit criteria and asked that the stratification methodology not be based on geography. In the nursing home Special Focus Facility (SFF) program, nursing homes from every state must be submitted for consideration and this method does not adequately address that there might be a preponderance of poor performing facilities in certain geographies.
For the SFP, CMS includes in the CY2024 Home Health Rule definitions, the criteria for selection and completion of the SFP, criteria for hospice termination from Medicare, and public reporting of the SFP. The SFP would commence as of the effective date of the final rule which would be January 1, 2024, and CMS anticipates selecting SFP hospices in CY 2024. CMS proposes to periodically review the effectiveness of the methodology and the algorithm of the SFP.
Relevant proposed definitions are:
- Hospice Special Focus Program: a program conducted by CMS to identify hospices as poor performers, based on defined quality indicators, in which CMS selects hospices for increased oversight to ensure that they meet Medicare requirements. Selected hospices either successfully complete the SFP or are terminated from the Medicare program.
- IDR: informal dispute resolution.
- SFP status: the status of a hospice provider in the SFP with respect to the provider’s standing in the SFP, which is indicated by one of the following status levels:
- Level 1 – in progress;
- Level 2 – completed successfully; or
- Level 3 – terminated from the Medicare program.
- SFP survey: refers to a standard survey as defined in §1105 and is performed after a hospice is selected for the SFP and is conducted every six months, up to three occurrences.
Identification Process
The SFP is for hospice programs that the secretary identified as having substantially failed to meet applicable requirements and would address issues that place hospice beneficiaries at risk for poor quality of care through increased oversight.
Unlike in the nursing home SFF program, CMS would identify a subset of 10% of hospice programs based on the highest aggregate scores determined by an algorithm. The hospices selected for the SFP from the 10% would be determined by CMS. Hospices with accrediting organization (AO) deemed status that are placed in the SFP would not retain deemed status and would be placed under CMS or, as needed, state agency (SA) oversight jurisdiction until completion of the SFP or termination.
Indicators used to identify “poor performance” would be:
- Survey reports
- Quality of Care Condition Level Deficiencies
- Substantiated Complaints
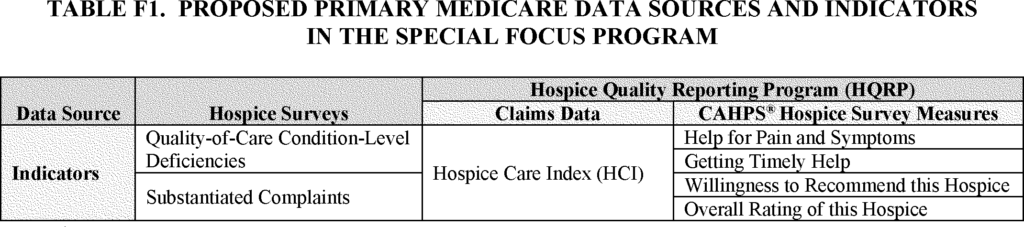
- Hospice Quality Reporting Program (HQRP) Data
- Hospice Care Index (HCI)
- CAHPS Hospice Survey
The quality-of-care condition level deficiencies (CLDs) are the 11 conditions of participation in the table below, which are the conditions of participation (CoPs) identified by CMS as being most closely tied to quality of care. CMS may explore incorporating other CoPs into the SFP methodology and is soliciting comments on an alternative approach that would do this.
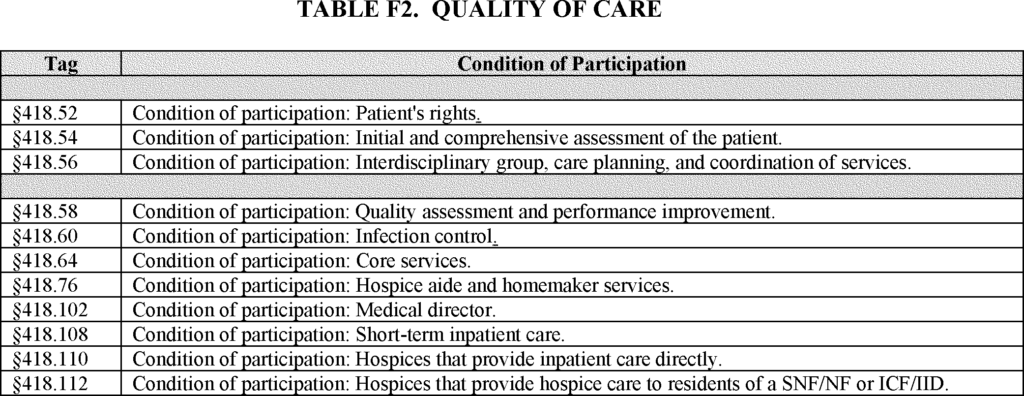
Quality of Care CoPs
CMS would count the total number of quality-of-care CLDs from the previous 3 consecutive years of data. In addition to quality-of-care CLDs, CMS proposes to include the total number of substantiated complaints received against a hospice in the last three consecutive years of data before the release of the SFP selection list.
- 88% of all SFP eligible hospices had no quality of care CLDs from CY2019-CY2021.
- 7% of hospices were not present (i.e., did not have a survey in that timeframe or results were not available)
CMS noted concern about the inter-surveyor reliability and state to state variability/AO variability.
- The technical expert panel, which provided feedback to CMS in 2023 on the development of a hospice SFP, still felt survey data important component of a SFP algorithm despite the limited number of CLDs.
- CMS notes that they have increased training for SAs and AOs on the hospice survey process.
In addition to the survey data, CMS would use HQRP data in the SFP algorithm. Specifically, CMS is proposing to include five publicly reported HQRP measures including the Hospice Care Index (HCI) overall score to identify poor performing hospices, as follows:
- Medicare claims-based measure: Hospice Care Index (HCI) Overall Score
- CAHPS Hospice Survey Data measures:
- Help for pain and symptoms
- Getting timely help
- Willingness to recommend this hospice
- Overall rating of this hospice
For the CAHPS Hospice Survey data, CMS proposes to use the adjusted bottom-box scores of the four measures above to create a CAHPS Hospice Survey Index. The bottom-box scores are used because they quantify reported problematic care experiences.
CMS would also make adjustments in the algorithm for hospices that do not have HQRP data. CMS is proposing to weight CAHPS higher than the other factors by multiplying the CAHPS index survey by two.
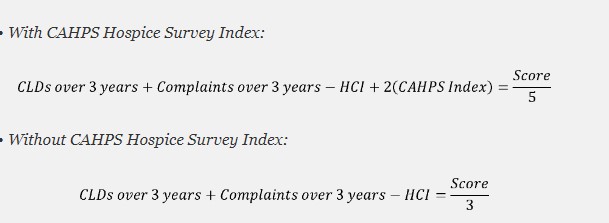
SFP CAHPS Calculation
Selection Process
The number of hospices selected to participate in the SFP would be determined in the first quarter of each calendar year. There will be no stratification criteria (e.g. not based on geography or size). The algorithm will be run yearly and establish an aggregate score from which SFP participants will be selected. CMS proposes to identify a subset of 10% of hospice programs based on the highest aggregate scores determined by the algorithm. The hospices selected for the SFP from the 10% would be determined by CMS.
Program Process
Those selected hospices would receive a recertification survey every six months. Additionally, SFP hospices would be subject to one or more enforcement remedies:
- Civil money penalties (CMP)
- Suspension of payment for all new patient admissions
- Temporary management of the hospice program
- Directed plan of correction
- Directed in-service training
When CMS chooses to apply one or more remedies, the remedies would be applied based on:
- noncompliance with one or more conditions of participation
- may be based on failure to correct previous deficiency findings as evidenced by repeat condition level deficiencies
- The enforcement remedies could be imposed for an SFP hospice with condition- level deficiencies on a SFP survey or complaint survey while in the program
If subsequent surveys also result in the citation of a condition-level deficiency or deficiencies for an SFP hospice, the enforcement remedies imposed could be of increasing severity. Increasing severity could mean a higher CMP than was imposed for the earlier noncompliance or increasing from one remedy to more than one remedy being imposed. CMS would use its discretion to determine what remedies are most appropriate given the survey results, and the hospice may be subject to remedies of increasing severity.
Completion, Graduation, Termination Process
To complete and graduate from the SFP, SFP hospices should, in an 18-month timeframe:
- Have no CLDs cited or Immediate Jeopardy’s (IJ’s) for any two six-month SFP surveys, and
- Have no pending complaint survey triaged at an IJ or condition level, or
- Has returned to substantial compliance with all requirements.
If there are complaint investigations or a 36-month recertification survey for a hospice while in the SFP, the SFP timeline may extend beyond the 18-month timeframe. A hospice in the SFP that fails any two SFP surveys, by having any CLDs on the surveys, in an 18-month period, or pending complaint investigations triaged at IJ or condition-level, would be considered for termination from the Medicare program. After completing the SFP, hospice programs would receive a one-year post SFP survey and then would start a new standard 36-month survey cycle.
Public Reporting
CMS proposes to publicly report, at least on an annual basis, the hospice programs selected for the SFP. Initially, this information would be posted on a CMS public-facing website, or a successor website.
The website would include general information, program guidance, a subset consisting of 10% of hospice programs based on the highest aggregate scores determined by the algorithm, and SFP selections from the 10% subset as determined by CMS, and the hospices’ SFP status.
Informal Dispute Resolution
In addition to the SFP, CMS is also proposing regulations to implement an informal dispute resolution (IDR) process to provide hospice programs an informal opportunity to resolve disputes related to condition-level survey findings for those hospice programs that are seeking recertification for continued participation in Medicare. IDR is meant to be an informal process whereby the provider has an opportunity to address the surveyor’s findings, either by disputing them or providing additional information. The proposed IDR for hospices is similar to the process already in existence for home health agencies. Some state survey agencies offer an IDR process to hospices even though they are not required to do so, and if the proposed IDR process is finalized some hospices may experience a change in the process.
The IDR process would be available to hospice programs to address disputes related to condition-level survey findings following a hospice program’s receipt of the official survey Statement of Deficiencies and Plan of Correction, Form CMS-2567, which is received after the survey has ended. CMS specified in the proposed rule that the IDR process may not be used to refute an enforcement action or selection into the SFP. Additionally, CMS proposes that the failure of CMS, or SA or the AO, as appropriate, to complete IDR must not delay the effective date of any enforcement action.
IDR may be initiated for programs under SA monitoring (either through a complaint investigation or validation survey) and those in the proposed SFP. For hospice programs deemed through a CMS-approved accrediting organization (AO), the AO would receive the IDR request from their deemed facility program, following the same process and coordinating with CMS regarding any enforcement actions. For deemed hospice programs, the AO communicates any condition-level findings to the applicable CMS Location. If a deemed hospice fails to meet the Medicare requirements or shows continued condition-level noncompliance, deemed status is generally removed and oversight is placed under the SA.
IDR Procedure
When survey findings indicate a condition-level deficiency (or deficiencies), the hospice program would be notified in writing of its opportunity to request an IDR for those deficiencies. This notice would be provided to the hospice program when the CMS-2567 Statement of Deficiencies and Plan of Correction is issued to the hospice. The hospice’s request for IDR would be submitted in writing (electronically or hard copy), include the specific survey findings that are disputed, and be submitted within the same 10 calendar days allowable for submitting an acceptable plan of correction. Results of the IDR would be one of the following:
- If any survey findings are revised or removed by the State or CMS based on IDR, and if CMS accepts the IDR results, the CMS-2567 would be revised accordingly. If CMS accepts the IDR results and the revised Form CMS-2567, then CMS would adjust any enforcement actions imposed solely due to those cited and revised deficiencies.
- If the survey findings are upheld by CMS or the state following IDR, the Form CMS-2567 would not be revised based on the IDR and there would not be adjustments to the enforcement actions.

 Shutdown Week Three: Impact of Ongoing Closure on Affordable Housing
Shutdown Week Three: Impact of Ongoing Closure on Affordable Housing